Opinion on proposed amendments to Listeria Regulation 2073/2005
On this page
Skip the menu of subheadings on this page.Advisory Committee on the Microbiological Safety of Food: Opinion on proposed amendments to Listeria Regulation 2073/2005
Date responses returned: 9th of August 2024
Report prepared by: Lauren Adams, Anthony Wilson (ACMSF Secretariat)
Executive summary
The Advisory Committee on the Microbiological Safety of Food (ACMSF) agrees that the scientific evidence base considered by the European Commission (EC) supports the need for legislative changes regarding Listeria monocytogenes (L. monocytogenes) in ready-to-eat (RTE) foods in the European Union (EU). An ageing population and increased listeriosis cases provide reasoning for why more stringent protocols are needed to ensure low bacterial counts.
The European Commission (EC) has published proposed changes to EU Regulation 2073/2005 Annex 1, Chapter 1, criterion 1.2; the food safety criteria for RTE foods which can support the growth of L. monocytogenes. The proposal stipulates that L. monocytogenes should not be detected in 25g up to the end of shelf-life of the ready-to-eat (RTE) product as opposed to the current requirement of before the food has left the immediate control of the food business.
The committee determined that the proposal should theoretically reduce disease levels, particularly among the elderly. However, the exact extent of reduction is uncertain. It is expected that the proposal will encourage food business operators (FBOs) to improve production, storage, and handling to minimise Listeria contamination. However, this is not without concern as smaller and medium sized FBOs could face economic burden due to the cost of evidence-based shelf-life studies, increased monitoring, and recall of contaminated product. Furthermore, FBOs cannot control products post-delivery, and issues such as temperature abuse at retail and consumer behaviour, can increase bacterial growth. There are also concerns over the validity of sampling and detection methods currently available for pathogenic L. monocytogenes.
Therefore, the proposed changes aim to enhance public safety by reducing Listeria contamination in RTE foods, but the proposal also presents significant challenges and financial implications for FBOs.
Introduction
In April 2024, the European Commission (EC) published proposed changes to EU Regulation 2073/2005 Annex 1, Chapter 1, criterion 1.2; the food safety criteria for ready to eat foods which can support the growth of L. monocytogenes. The proposals were adopted on 3rd July by the Standing Committee on Plants, Animals, Food and Feed (SCOPAFF) and will move to consultation with the European Parliament.
The requirement to adopt Regulation (EC) 2073/2005 is contained within Regulation (EC) No. 852/2004 which is listed in Annex II of the Windsor Framework and so is directly applicable in Northern Ireland.
The proposed amendment is to the second part of criterion 1.2. It proposes L. monocytogenes should not be detected in 25g up to the end of shelf-life of the ready-to-eat (RTE) product as opposed to the current requirement of before the food has left the immediate control of the food business. The amended criterion will continue to be linked to the first part of criterion 1.2 and apply when the manufacturer is unable to demonstrate that the product will not exceed 100 colony forming units (cfu) per gram of food (cfu/g) throughout the shelf-life.
The evidence bases for introducing this legislative change is a reported upsurge in listeriosis cases, as well as aiming to align EU rules with international ‘Codex Alimentarius’ standards on the acceptable level of contamination by L. monocytogenes of certain categories of RTE food sold on the EU market.
The latest report on zoonoses(1) from the European Food Safety Authority (EFSA) has reported that cases of listeriosis in humans have increased in the Union by 15.9% from 2021 to 2022. The Authority also reported that the number of deaths in 2022 caused by L. monocytogenes is one of the highest in the last decade.
| Zoonosis | Number of cases | Notification rates | |||
| Year | 2022 | 2021 (absolute difference) | 2022 | 2021 (absolute difference) | 2022-2021 (relative difference) |
| Listeriosis | 2738 | 373 | 0.62 |
+0.08 |
+15.9 |
Methodology
The Advisory Committee on the Microbiological Safety of Food (ACMSF) secretariat formulated three questions for members to provide evidence-based opinions regarding the proposed amendment to the second part of criterion 1.2.
- Is the need for change justified? What are your views on the evidence cited in the latest report on zoonoses as the basis for the changes being introduced in this proposal?
- Is the change likely to have the intended effect? If implemented, what are your views on the effect this change may have on disease burden and death rates?
- What other implications could the change have? Based upon your expertise, (i) what knowledge and practical approaches will food business operators have to develop to meet the new proposed requirements, and (ii) what wider capacity will FBOs need to access to implement such approaches and is this available (e.g., sampling, testing, and modelling)?
Responses were received from ACMSF members.
Results and discussion
Proposed change to legislation is justified by the evidence base.
Members who responded largely agreed that the change in legislation is justified by the evidence base. Below are the responses provided by individual members.
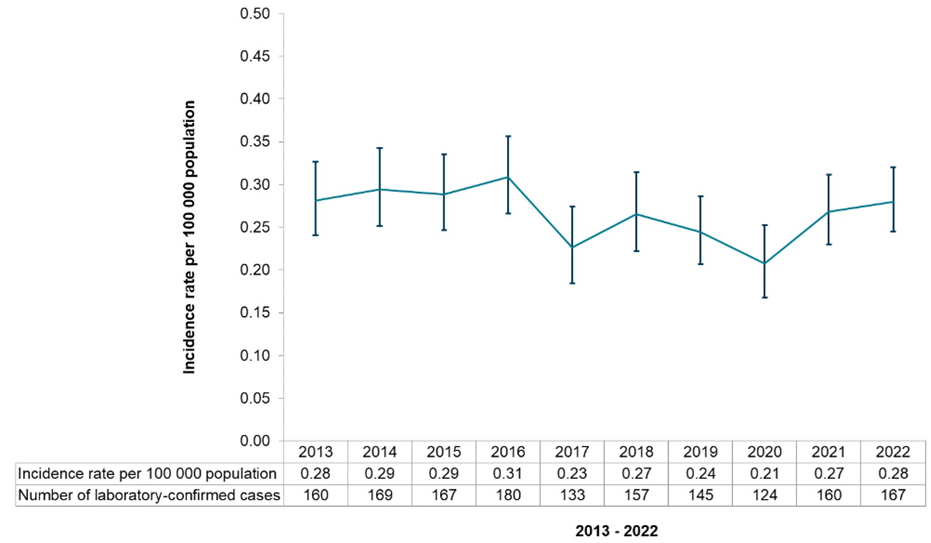
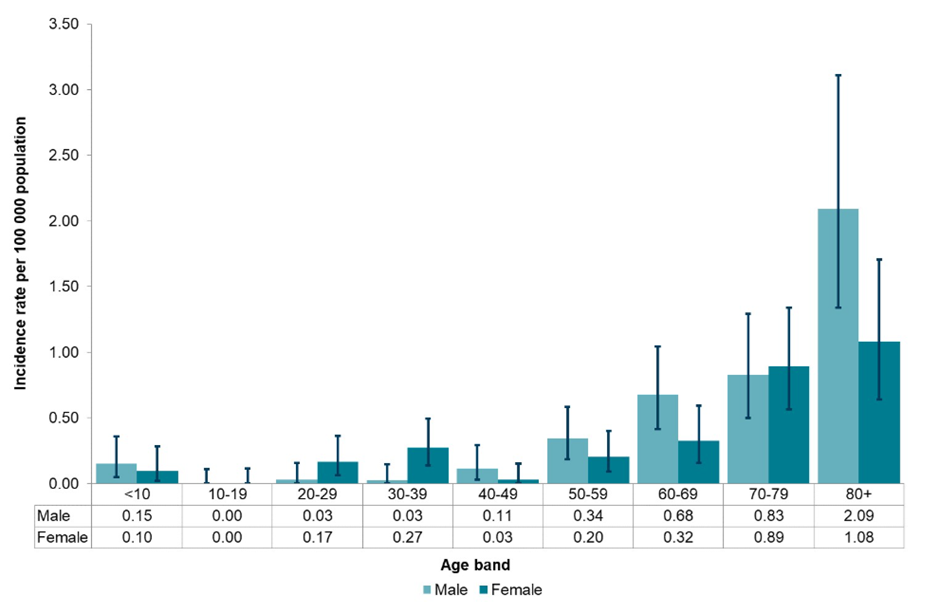
Response 1: The epidemiological data released by PHE2 indicates that the increase in numbers of listeriosis cases in the UK over the same period has not been as dramatic (6.4% increase compared to the previous five years median; see Figure 1). However, an analysis of the population structure shows that the elderly (over 70) are the highest risk group (Figure 2).
The number of people aged 85+ years was estimated to be 1.7 million in 2020 (2.5% of the UK population) but is projected to increase to 4.3% of the UK population by (3.1 million; ONS data) by 2045. On this basis, with no other change in risk factors, we would predict a nearly 2-fold increase in the number of cases in this age group alone over the next 20 years and therefore is should be a government priority to try and reduce risk of infection in anticipation of this.
Figure 1 Annual cases and crude incidence rate of listeriosis reported in England and Wales, 2013 to 2022 (UKHSA)
Figure 2 Age-specific incidence of listeriosis in England and Wales, stratified by sex, 2022 (UKHSA)
Response 2: The report makes clear there has been a worrying increase in the number of Listeriosis cases and deaths in 2022 compared with 2021, and the evidence suggests ready to eat foods, particularly those including fish products, have been associated with these. It also notes the age groups affected are those over 65 years, who I consider may not be in the so-called 'vulnerable' category that would be covered by other legislation. Thus, review and amendment of the legislation is justified; the change proposed is sensible as it extends the manufacturers' responsibility across the full shelf life of RTE products and so will encourage them to provide suitable packaging and labelling to minimise the risk of listeriosis arising from consumption of the product during the entire period in which it is expected to be consumed.
Response 3: I think the report does justify the need for change and has information to support this, namely the worrying increase in the number of cases reported and high rate of hospitalisations, and elevated morbidity and mortality, which is particularly noted among elderly people. Also, the increased number of outbreaks contributing to these increased figures
Response 4: The need for change appears to be justified as it appears to be an increase in listeriosis cases in the EU. This increase in cases is also seen in other countries, such as the US with on-going multistate outbreaks (July 2024). If the data reflects a rising trend or insufficient control over Listeria in RTE foods, tightening the criteria makes sense.
Also, the alignment with Codex Alimentarius standards suggests the EU is aiming to not only handle internal public health concerns but also to strengthen its own standards which could improve market access for EU products and ensure a higher standard of food safety.
Response 5: In 2022, there was a progressive increase of cases of listeriosis (+15.8% vs. 2021), which could be partially explained by the increase of outbreak cases specifically in Austria, Belgium, Denmark, Finland, Germany, Hungary, Italy and Spain. However, the general EU trend for listeriosis in 2018–2022 showed no significant increase or decrease, except for some MSs (Austria, Denmark, France and Hungary) with a significant increase, and for Estonia with a statistically relevant decrease.
This is not sufficient evidence to justify the proposal changes. It will, however, raise knowledge of Regulation (EC) No 2073/2005 in general, which is essential.
Response 6: The epidemiological data represented in ‘Listeria in England and Wales: Summary for 2022 published by UKHSA is supportive of the proposal
Response 7: The tolerance of low levels of L. monocytogenes in foods, as the Draft regulation document states, is based on the ‘scientifically recognised that only ingestion of food containing concentration of L. monocytogenes over the limit of 100 cfu/g is potentially injurious to health’. However, there have been a number of studies examining the L. monocytogenes load within Ready-To-Eat food products implicated in outbreaks of disease. This has provided evidence that in some cases, the burden of L. monocytogenes within the food product has been below the 100 cfu/g or ml threshold. For example:
- An outbreak in the US in 2015, linked to contaminated ice-cream (a product assumed to not support the growth of Listeria), found that 92% of samples produced around the time of the outbreak on the implicated production line had <20 MPN/g
- A study detailing an outbreak of listeriosis linked to butter in Finland in 1998-1999 (a product which multiple studies have shown can support the growth of L. monocytogenes, e.g. https://doi.org/10.1111/j.1471-0307.2009.00505.x), hypothesised that prolonged exposure to a food contaminated with low levels of L. monocytogenes may have caused the outbreak infections (https://doi.org/10.1016/S0168-1605(01)00532-3).
Although these involve ‘at risk’ groups, such cases in a hospital or care setting, for example, could be exposed through foods not specifically manufactured for infants or special medical cases. In the case of foods that would support the growth of L. monocytogenes, temperature abuse is also a relevant concern here, which could occur at various points through the life of the food product. Based on the above, I would support the proposed amendment, as reasoned by item (5) within the Draft regulation document.
Proposed amendments should theoretically reduce listeriosis cases
Response 1: There are two sets of standards relating to Listeria for food producers which relate to: (a) those products intended for highly vulnerable groups (specifically those intended for infants and for special medical purposes) and (b) those intended for consumption by the general product. For category (a) products, the regulations state that Listeria should not be detected in the product, based on 25 g samples, and this proposed change in regulation does not affect these category (a) products.
However, for category (b) products manufacturers are currently allowed to release product if it contains a low levels of L. monocytogenes that is not predicted to exceed 100 cfu per g at the end of their shelf life. For products that will not support the growth of this organism (i.e. the intrinsic properties of the food means that bacterial numbers cannot increase during shelf life), this is a very straight forward pass/fail situation – if the product is shown to have less than 100 cfu/g, it can be released for sale. This proposed change in regulation does not affect these types of category (b) products.
For category (b) products that will support the growth of Listeria, the current regulations state that the product can only be released if there is evidence that the levels of L. monocytogenes in the product will not exceed the limit of 100 cfu/g throughout the shelf-life. This limit is set because there is evidence to support the fact that ingestion of foods by healthy adults containing less than 100 cfu/g is very low risk.
The difficulty for manufacturers is that the models used to predict whether these limits would be exceeded need to include events that occur after the product has left the control of the producer. A key factor that needs to be considered is temperature abuse during storage - and this introduces a large element of uncertainty into the risk calculations.
This is illustrated by the evidence that low level contamination combined with inadequate temperature control contributed to a number of listeriosis outbreaks in UK hospitals (PHE report, 20203) and is reflected in the emphasis on the need for good temperature control of foods in guidance to reduce the risk of listeriosis in health care settings (FSA, 20164).
This uncertainty appears to be the driver behind the proposed change in regulations; it recognises the fact that it is very difficult to model all possible storage scenarios and then to define what a safe level of L. monocytogenes would be in a product in on release if this organism is then able to grow.
The new regulation is more stringent in that it states that category (b) products can only be released if L. monocytogenes is detected where evidence can be provided that the organism could never reach 100 cfu/g during its shelf life – irrespective of the storage conditions used/temperature abuse after the product has left the immediate control of the producing food business operator. It states that in the absence of such evidence, the stricter criteria should be applied, and no L. monocytogenes should be detected in 25 g of product before release.
It is difficult to directly predict the effect of these changes on disease burden, but any measure that reduces the risk of the consumer ingesting food products that contain significant levels of L. monocytogenes would be predicted to reduce levels of disease, particularly in the elderly – and, of course, this is the group where we expect to see an increase in the number of cases based just on predicted changes in population structure.
It has also been reported that increases in the cost of living have driven two changes in consumer behaviour (a) a tendency to keep food for longer, including beyond recommended shelf life, and (b) reducing electricity usage by increasing the temperature of domestic fridges/turning them off to save money. Clearly both of these behaviours would increase the risk of levels of Listeria in category (b) products exceeding safe levels. Therefore, measures to reduce the likelihood of L. monocytogenes being present in category (b) products would also be predicted to have a positive effect in reducing disease burden.
Response 2: It is not clear from the evidence presented if the change would have the intended effect, but it seems likely that it should. The outbreaks reported have often been traced back to RTE products and these were most likely consumed during their shelf life but after they had left the immediate control of the FBO, and there has previously been nothing in the legislation to require any specific Listeria controls beyond this point. Introducing this additional requirement should encourage the FBOs to seek to manage the production, storage and handling of their products such that the risk of Listeria contamination at an infectious dose level is minimised.
Response 3: Changing the criteria and having a 2-tier system for those businesses that can demonstrate to the satisfaction of the competent authority that the level of L. monocytogenes will not exceed the limit of 100 cfu throughout the shelf life of the food, and a zero tolerance for those that can’t, will assist officers with enforcement.
Larger businesses who have technical teams and expertise to conduct shelf-life studies will set shelf life according to the evidence from the study, so there will be no change for those large manufacturers.
The changes could have the desired effect of tightening controls where there may be a lack of knowledge or control around Listeria with those businesses posing a higher risk. Therefore, raising standards of compliance requiring businesses to either invest in evidence-based shelf-life studies or a stricter, zero tolerance criteria for such products with an inadequate evidence base.
There are many small and medium sized businesses that do not have sufficient expertise in this area, and this is not always considered and not always picked up on inspections during routine visits. Many businesses do not conduct robust shelf-life studies and often rely on poorly trained consultants to act as experts to determine shelf-life limits and to determine if products can support the growth of Listeria.
Changes in this legislation will highlight this issue and create a focus on this area during the inspection process. I would hope this would have the desired effect of raising standards of Listeria control, awareness and an increase in monitoring and sampling to identify products that may be non-compliant. This will result in identifying issues in a timely manner and thus, removing contaminated foods from the market more efficiently once a positive result is found, therefore reducing the risk of such food being consumed and causing illness and possible death.
Response 4: In principle, making it mandatory for no L. monocytogenes to be detected in 25g of the product up to the end of its shelf-life should theoretically reduce the incidence of listeriosis among the population. Early and strict controls can significantly reduce the number of cases from foodborne diseases.
However, the impact may be somehow limited as this adjustment does not specify a quantity before foods leave immediate control of the FBO and that the sampling should be representative of the foods produced by the FBO. The infectious dose of L. monocytogenes can be very low, and the organism is quite resistant and can survive various preservation and storage conditions. In addition, a decrease in cases may not reflect a decrease in mortality from listeriosis, particularly affecting vulnerable groups, which can limit the impact of this change.
Response 5: I do not believe it will have much of an effect. The FBO is not in control of the products once it is delivered – either to another manufacturer in the case of RTE ingredients; or retailer; or distribution centre. The point of temperature abuse, a considerable industry issue, not just at manufacturing site, but at retail display, especially the grab-and-go fridges during summer or power cuts.
Adding further suggested reasons for my opinion: the proposal changes require the evidence of absence or < 100 cfu/g at the end of shelf life by the manufacturer. The end of shelf life is indicated by the UB date on pack for pre-packed products. In the event of RTE ingredients for catering or further manufacturing use (e.g. smoked salmon, cooked sliced cheese, cooked sliced ham, hummus) the secondary manufacturer would have both an UB date on pack as well as ‘once opened use within x days. Microbiological quality of product is usually demonstrated based on the on pack UB date, results provided by one of the many laboratories that provide this service. The manufacturer has no control over what happens if the pack is opened elsewhere (e.g. deli or manufacturer of sandwiches and salads for retail). Environmental contamination of the products in this open pack frequently occurs if there is a low-grade presence of L. monocytogenes (biofilm formation) in the factory – chopping boards, knives, surfaces in direct contact with food, staff hands etc. Knowledge of microbiology in food manufacturing premises is very poor, especially the nature of biofilm formation and cleaning chemicals. In addition, the sandwich/salad manufacturer may use the product very close to the end of its on pack UB date, put it in one of their products which gets a UB date that frequently exceeds the raw material UB date. I see these examples very frequently, as well as the increased frequency of frozen RTE ingredients. Whole brie cheeses, sliced cooked meats, smoked salmon is purchased at a lower price in bulk close to the on pack UB date by brokers and distributors, frozen down and sold as a frozen product months past the chilled UB date – of particular risk to L. monocytogenes growth and/or survival. Not illegal, as they put a ‘frozen on date’ with a new frozen shelf life – use within x amount of time, defrost before use.
Based on present FSA guidance (attached for those who have never needed to engage with it), a food product is unable to support the growth of L. monocytogenes if the food is made for consumption within 5 days of preparation. In general, this means that all components of the product must comply with the ‘within 5 days of preparation’. This is more often than not ignored. For example, a batch of tuna mayonnaise is prepared on a Monday and used in salads, sandwiches and wraps that carry a UB date of p + 4 (4 days after production). This mean the product, according to guidance, does not support the growth of L. monocytogenes and therefore need not be tested. However, this batch of tuna mayonnaise could still be in use on Wednesday, meaning Wednesday’s finished products will have an ingredient with a ‘6 days after production’ age at the end of the product’s shelf life and therefor the product should be considered for study to determine if it is able to support the growth of L. monocytogenes.
Response 6: The construction of the existing regulatory position on the criteria and standards is that the proposal would be most relevant to products where the intrinsic (and possibly certain extrinsic factors) had not been validated to arrest multiplication to below 100 cfus during and throughout shelf life. There are two related challenges:
- FBO Validation of Controlling Factors according to events that fall under their control which can be problematic, with that problem being compounded by attempting to predict the effect of events that are beyond their Control. Temperature (and time) abuse in the supply chain and by the consumer is here significant.
And
- The current capability of Official Controls carried out by Local Authorities to verify compliance with any new regulatory requirement must be ensured. Predictably work would have to be undertaken on the Methods and Techniques required by Regulation (EU) 2017/625 of the European Parliament and of the Council on Official Control in order that Environmental Health Depts (and Third-Party Auditors) were supported in being able to effectively verify compliance with such a new regulatory position.
Both challenges would have to be met in order that the new regulatory would be effective.
Response 7: It’s difficult to quantify or predict if this will have a significant impact on the burden of disease. Many food businesses do not establish validated growth prediction models for their products, due the costs and product specificity (i.e. having to conduct individual challenge trials for multiple different products/formulations). But given evidence suggests contamination levels below 100 cfu/g may still lead to onset of disease, there is potential for this disease burden to be reduced; how significant or otherwise this reduction could be, is hard to predict. It would require analysis of data on the proportion of incidents that would be impacted by this category, and data on how these fits within incidences of disease.
Forecasting future implications that may arise after introducing the proposed amendment
Response 1: This change will have an impact on producers of short shelf-life foods because it is almost impossible to model the growth of an organism when key environmental factors, such as temperature, are unknown.
This category includes many short shelf-life RTE products, such as sandwiches, pre-prepared salads and fresh cheeses, but also includes processed meat and fish products, other non-fermented dairy products and pre-prepared fruit products. The issue for manufactures is that the ISO 11290-2 method used to detect low levels of L. monocytogenes typically takes 5 days before a confirmed result is achieved.
Unfortunately, all these RTE foods may be contaminated with other 28 species of Listeria, and all but one of these are non-pathogenic and are not a risk to human health. The isolation method will allow the growth of all 28 species, and therefore the detection of growth alone is not sufficient to determine whether a product is safe to release. Hence, the change in these regulations mean that the detection of low levels of Listeria species (i.e. any member of the genus) will result in more products being held while confirmatory tests are performed, whereas previously - if levels were below a threshold - the product could have been released.
Therefore, this change will have an economic impact on producers of category (b) products that will support growth of Listeria by reducing shelf life of product.
It is difficult to predict what practical approaches food business operators can develop to mitigate against these problems.
- Improved modelling may allow them to eventually provide evidence to identify safe levels in their food products, but it is unlikely that this will be a solution that is available to them in the short term.
- Often the introduction of new molecular tests to screen food products can be used as part of testing programs, but it will take time for these processes to be validated.
- New sampling programs could be developed to provide more data for predicting growth levels, but again it will take time for these processes to be validated, and these would also be very product-specific requiring significant investment of time by the producers.
Response 2: I would expect these additional requirements to impact on the preparation, packaging and labelling of their RTE products, and require producers to work with their distributors and retail merchants to ensure that RTE foods are correctly stored (e.g. cold chain requirements).
Response 3: I don’t think there will be any change for larger businesses as the changes to the legislation will have no effect as these will have systems in place to demonstrate the limit will not be exceeded so they will remain on the existing legislative requirement of 100 cfu during shelf life of the product.
However, I think there will be an initial burden on smaller and medium sized businesses to undertake such studies which will be a financial burden on them. There is a lack of expertise within these smaller businesses who are then forced to buy in help in the form of consultants. These consultants, in my experience and from speaking to other officers around the country, often offer poor quality advice and are not adequately trained to advise in such matters. Poor advice can lead to shelf-life studies being conducted incorrectly, wasting the businesses money and not providing the business with meaningful results or setting an unreliable or potentially unsafe shelf life.
It may also result in the reduction of shelf-life following studies when completed properly and business may suffer losses as those caterers/retailers they supply are often wanting extended shelf life due to consumer demand.
There is also an additional cost in the anticipated increase in sampling for businesses. This is something that I have seen declining due to increased costs at the labs, and as part of a cost cutting exercise for those businesses that are feeling the effects of the increasing costs of energy and food.
I anticipate that there will be a reduction in sampling by the smaller manufacturers if more stringent limits are set. If little or no verification sampling is carried out, then little or no adverse results are found and therefore there will be no product recall or withdrawal. Any action such as a recall has an impact on the businesses reputation as well as the cost involved in the logistics of removing the products and the associated waste costs. I have also had experience and had conversations with private labs where I understand positive results are found but this information is not always provided to the competent authority or acted upon by the business.
Response 4: Other implications for the FBOs:
- Knowledge and practical approaches:
- Testing and Monitoring: FBOs will need to refine their testing protocols to detect any presence of L. monocytogenes through more frequent sampling. As FBOs are already testing for Listeria, this change should have minimal impact on the knowledge capacity.
- Process Control: Adapting existing processes in production would have small impact unless Listeria is detected when otherwise it would not have been to ensure that conditions that might favour Listeria growth are minimised.
- Capacity Needs:
- Technological: Enhanced capabilities for microbial testing and real-time data collection concerning food safety parameters along the supply chain may be necessary.
- Logistical: some impact to maintain product compliance up to the point of sale and enhanced traceability systems and recall procedures.
Financial: There might be some costs involved in updating equipment and logistical needs above, which could impact smaller operators more significantly.
Response 5: The new proposal is a ‘simple’ quantification amendment, consequently one assumes mere understanding of the new cfu/g limits would suffice, with addition of financial investment to obtain the required evidence.
However, knowledge and understanding of (EC) No 2073/2005 (not amended) is presently extremely poor, even in factories with Industry Accreditation.
Food science/technology/microbiology knowledge will need to be developed. Firstly, if a product has (a) a pH of less than or equal to 4.4, or (b) Aw is less than or equal to 0.92, or (c) pH is less than or equal to 5.0 and the Aw is less than or equal to 0.94, it is considered not able to support the growth of L. monocytogenes. Taking pH and Aw measurements on site requires knowledge and significant investment in equipment. Otherwise, products can be sent to laboratories for such measurements. However, knowledge and expertise of sampling methodology in preparing these samples to be sent to the laboratory is needed. For example, I provide results from a recent study I conducted on a catering pack of cooked streaky bacon:
- aw 0.84 (15.5°C)
- aw 0.84 (15.8°C)
- aw 0.87 (16.0°C)
- Preservatives: E301 sodium ascorbate; E250 sodium nitrate
- Salt % = 3.4
- aw 0.95% (15°C)
- aw 0.94% (15.2°C)
- aw 0.91% (15.7°C)
1 – 3 measurements were taken from the fat-only parts sampled from slices from top, middle and bottom; 6 - 7 measurements were taken from the meat-only parts (from 3 different slices from top, middle and bottom of pack).
Anyone without the scientific knowledge would simply take a few rashers from the top and not separate fat from meat for sampling, nor would they request from the laboratory to separate the fat from the meat for sampling. In addition, research (although in ham, and not bacon) demonstrates the combination of E301 and E250 does not impact the growth of L. innocua, and E250 does not impact the growth of L. innocua in deeper areas of the meat (Hospital et al, 2017). It is often accepted in industry the presence of these preservatives demonstrates an inability of presence. Two out of 3 sample dates (3 separate times/batches/dates) sent to ALS Laboratory came back positive for the presence of L. monocytogenes in 25g per product of this bacon product since November 2023.
Emulsion type mixes in RTE products (mayonnaise mixes) can be another challenge. From the same study:
Egg mayonnaise mix
Six different samples were taken, three from the same batch straight after mix production, 1 - 3 (25 January 2024) and three during assembly production, 5 - 7 (31 January 2024). Samples were taken from various areas and depths of each batch tray. All samples were kept in ambient conditions during testing.
- pH 4.40 (11.9°C)
- pH 4.14 (12°C)
- pH 5.63 (14.2°C)*
- Salt% = 0.2
- pH 4.47 (9.6°C)
- pH 5.08 (9.0°C)
- pH 4.40 (9.7°C)
* sampled from the bottom of the tray with signs of insufficient mixing; sample was mostly plain egg with very little mayonnaise
Anyone without the scientific knowledge would simply sample from the top of a container. In addition, decimal point pH meters are very expensive. Standard pH meters used in food factories are often ones that only measures one decimal point and consequently a pH 4.47 would be missed.
Additional labelling requirements? What is the meaning of ‘end of shelf life’? Using the example of frozen down bulk buy of RTE ingredients by brokers and distributors close to the end of its chilled on-pack UB date. Who is responsible for generating the evidence required by the new proposal in such a scenario? If the manufacturers of these products add a labelling disclaimer ‘not suitable for freezing’, such products end up as food waste with environmental and food security considerations.
In my experience, FBO’s that I have been inspecting the past 17 years, have always provided the microbiological safety of their products to the end of the on-pack stated UB date, including frozen foods. Never have I come across data that only relates to L. monocytogenes at the point of product dispatch, these are the requirements of the Industry Accreditation Standards that I audit against. The zoonoses report does not provide detail of historic outbreaks in terms of source of products. Pre-packed products v those from deli counters and restaurants. My experience is within the pre-packed food product industry.
Considering the burden of inspecting compliance to the new proposals should also be taken into consideration.
Response 6: Validation of Controlling Factors/Measures by FBOs. This is a complex and challenging position for FBOs to find themselves in. The larger and better resourced FBOs may be able to access the necessary technical resources, but smaller FBOs such as artisanal cold smoked fish producers for example will not be so able. Predictably many FBOs will not be able to comply because validation is beyond their resources and/or they will default to the criteria of no L. monocytogenes being detected in 25 g of product. In this latter option they will face further challenges resulting from the expense of verification microbiological assays and the inability of suppliers to guarantee the absence of L. monocytogenes, albeit they will be trying to rely on that approach as part of their approach to compliance. The Scottish cold smoked salmon and the UK-wide raw cheese industries will be caught by this challenge. N.B the current proposition of raw cheese making FBOs is for the incoming milk to be of such a microbiological standard that it will not cause adverse health effects even with the absence of downstream (i.e. in process) Controlling Factors and Measures. The proposed regulatory changes for L. monocytogenes may drive the need to validate the downstream controls. (It is noteworthy that recent data has cast doubt on the proposition of clean milk. Most of the concern was in relation to E. coli spp although L. monocytogenes does feature. The occurrence of E. coli on the raw milk infers the potential for contamination with L. monocytogenes and subsequent multiplication in a process that lacks downstream Controlling Factors and Measures. (Refer to McLauchlin et al (2020) attached hereto).
- The general principal of hazard control is usually to attempt to control at source. In addition to in-product Controlling Factors and Measures such approaches are and should further be explored. The proposed regulatory change may drive this? As stated above most FBOs will predictably default to the no L. monocytogenes being detected in 25 g of product approach, but further work will likely have to ensue in the supply chain. The aquaculture sector is considering re-locating fish farms further offshore i.e. into deeper water with less land-based influent where L. monocytogenes levels are lower, and dilution factors are greater. Novel approaches have been attempted in relation to disinfecting fish within the slaughter water and include chemical and acoustic approaches.1-2 log reductions have been recorded and although these are relatively modest, they are occurring at the very beginning of the process inferring subsequent levels (all being equal) will be lower and easier to eliminate or control within the boundaries of microbiological criteria. The Raw Cheese industry may soon face the challenge of Validation.
Response 7: FBOs will likely need to have more engagement/control of their products associated supply chains (transit conditions, temperature control, etc). after loss of immediate control when the product leaves the processing facility.
Modelling/challenge trials would be useful, but generally, these are cost-prohibitive for many businesses.
Additional considerations
Response 1:
- As stated earlier, the genus Listeria now contains 28 different species, and 26 of these lack the virulence genes required to infect either man or animals (the other pathogenic species is Listeria ivanovii, but this is very rarely isolated from foods). These organisms are primarily environmental saprophytes, living in soil, but can survive and persist in food factory environments. Hence, contamination of RTE products by members of the Listeria genus is not uncommon, resulting from contamination of fresh produce in the field or even contamination of products during manufacture within the food factory environment.
There have been suggestions that the regulations should not permit the detection of any Listeria species in high-risk foods on the grounds that the presence of these organisms’ act as an indicator that L. monocytogenes may also be present. However, there is a lot of evidence that presence of Listeria species other than L. monocytogenes is not a good predictor for the presence of this pathogen.
Therefore, a drive towards even more stringent criteria is not supported by the scientific evidence.
- When considering the introduction of new molecular tests for screening foods, there are two issues for food producers:
- Dead cells in food products will still give a positive PCR (or other molecular detection) signal; for methods to be meaningful in the context of food products detection of DNA alone is not sufficient it indicates the presence of a viable organism that might cause infection.
Therefore, any molecular methods considered for screening foods should include a methodology that confirms that the cells detected are alive. There are new commercial methods being developed that meet this criterion.
- Detection of a gene alone is not sufficient to determine phenotype; there are a growing number of reports that there is a selective pressure for L. monocytogenes growing in the environment to lose the virulence genes that they require to be pathogenic.
These strains, when present in a food sample and plated on selective agars, would be identified as belonging to one of the non-pathogenic species, such as L. innocua and, as the strains are not able to infect human cells, this is not a problem. However, molecular screening tests cannot distinguish between L. monocytogenes strains that contain these genes and are virulent and the non-haemolytic, non-pathogenic variants.
Therefore, when considering introducing molecular screening tests, it should be noted that genotypic screening alone may result in more test failures.
Response 2: There is no recognition of the differing virulence of L. monocytogenes strains which I understand is considered when a product is found to be contaminated or there is an outbreak. I believe this can communicated from the FSA or other professionals in the risk management advice given when considering action to be taken and for consideration when recalling/withdrawing product from market.
With the existing 100 cfu limit during shelf life, when there is a positive result from an initial test from a sample from a lot/batch during the shelf life, there is a significant delay in a decision being made for action, as results take some time to come through due to awaiting the enumeration process (7-10 days) allowing the potentially contaminated food to continue to be placed on the market for consumption. Having a zero tolerance for such products from some businesses will allow a more rapid recall or withdrawal of the contaminated products protecting public safety. I know that some businesses recall as a matter of precaution without the full enumeration results but in my experience, there have been delays while awaiting confirmation of a result above 100 cfu allowing unsafe product to continue the market until results have been received.
Conclusion
The need for change in legislation regarding L. monocytogenes in RTE foods is generally considered to be justified by members of the ACMSF, based on their appraisal of the evidence base considered by the EC. The evidence of an ageing population, with 4.3% expected to be over 85 by 2045, compared to 2.5% in 2020, predicts an increase in cases within this vulnerable group. The rise in listeriosis cases in 2022, associated with RTE foods, further supports the need for more stringent protocols to ensure low bacterial counts and reduce the number of cases.
The proposed changes should theoretically have the intended effect of reducing disease levels, particularly among the elderly, although the exact extent of reduction is uncertain. The new regulation, which affects category (b) products that support the growth of L. monocytogenes, aims to ensure that the organism does not reach 100 cfu/g during shelf life. Applying stricter criteria, such as L. monocytogenes detected in 25 g of product before release, is likely to reduce exposure. However, a decrease in cases may not necessarily translate to a decrease in mortality rates.
The additional requirements are expected to encourage FBOs to improve production, storage, and handling of practices to minimise Listeria contamination. This will likely necessitate investment in evidence-based shelf-life studies and increase monitoring, helping to identify and remove contaminated foods more efficiently. However, FBOs cannot control products post-delivery, and issues like temperature abuse during dispatch and retail, as well as consumer behaviour, may impact the effectiveness of the legislation.
The ISO 11290-2 method for detecting low levels of L. monocytogenes can take up to 5 days for confirmation, potentially delaying product release and impacting manufactures’ economically. This will likely affect smaller and medium-sized businesses due to the cost of shelf-life studies, sampling, and product being recalled or not released. The proposal will also increase the burden of compliance on inspectors to enforce the legislation, as they may need support in effectively verifying compliance with such a new regulatory position.
Overall, while the proposed changes aim to enhance public safety by reducing Listeria contamination in RTE foods, they also present challenges and financial implications for food businesses.